Research News: How Much Do Cells Know?
AI and physics provide new insight into cellular communications
By Prof. Purushottam Dixit
In multicellular organisms like humans, many centrally secreted signals instruct individual cells to carry out specific tasks. One such example is insulin. Insulin is secreted by the pancreas in response to a rise in blood glucose levels, resulting from food intake. High insulin levels instruct cells (for example, the skeletal muscle cells) to eat up the glucose in the blood. Importantly, the strength of the signal can dictate how vigorously cells carry out the instructed task.
In general, there are thousands of signaling molecules circulating in our bodies, telling our cells what to do next, affecting important decisions in metabolism, immune response, and wound healing. It is therefore imperative that our cells sense the change in extracellular signaling molecules and act accordingly. The task of deciphering chemical variations in the extracellular environment and carrying out appropriate downstream actions (for example, eating up glucose after sensing insulin) is carried out by the so-called signaling networks; networks of biochemical reactions that involve chemical modifications of different proteins in response to dynamical changes in cellular environment.
Dynamics of biochemical networks are governed by rare processes at small length scales and are therefore dominated by thermal noise. Consequently, cells’ responses to the same signal can vary substantially in a population of genetically identical cells. The rigorous mathematical framework to quantify the amount of information present in cells’ signaling networks is information theory, developed by Claude Shannon in the 1940s and ’50s. This theory imagines the signaling network as a channel that transmits information about the extracellular signal (input) to intracellular decision-making machinery (outputs), such as insulin to glucose uptake. The channel capacity of a signaling network is the maximum amount of information (in bits) that can be transmitted. Recent experimental advances in measuring protein concentrations at the single-cell level allow us to quantify the channel capacities of many signaling networks in humans. Surprisingly, in most cases, the channel capacity is estimated to be quite small, around one bit. This suggests that human cells can, at best, detect the presence or the absence of the signal but not the strength of the signal.
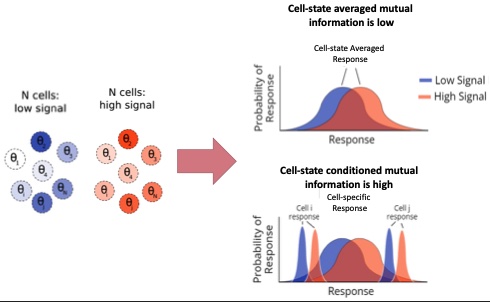
Top right: There is a significant overlap in experimentally observed cellular responses across different input conditions. This suggests that cells may be unable to distinguish between the two input signals. Bottom right: At the single-cell level, the responses corresponding to the two inputs are quite well-resolved, suggesting that single cells can, in fact, distinguish between different inputs.
This is surprising because human cells are known to perform complex tasks in response to subtle changes in their environment. Our laboratory, with students Andrew Goetz and Hoda Akl, decided to tackle this conundrum. Recognizing that the observed variability in cells’ output arises from a combination of stochastic fluctuations at the single cell level as well as persistent variability in cell state variables (cell size, cell cycle state, levels of different proteins, and so on), we concluded that to quantify how much individual cells know about their environment, we need to disentangle these two contributors.
Our group was recently awarded the Maximizing Investigator’s Research Award (MIRA) by the National Institute of Health (NIH) for developing artificial intelligence methods that combine physics-based models of signaling networks with single-cell data. As part of that sponsored research, our team developed AI algorithms to disentangle different sources of noise and variability at the single-cell level. We developed novel information-theoretic methods that explicitly account for differences in cell states and applied the developed AI algorithms to study several human signaling networks. One of the main findings is that individual cells know much more about their environment than what a population-based analysis would indicate. For example, we showed that the insulin-like growth factor signaling network (closely related to the insulin signaling network) can transmit on an average up to 3-4 bits of information about extracellular input. Importantly, we also showed that individual cells have differing abilities to sense their environment. Moreover, new computational methods allowed us to pinpoint the biochemical parameters that allow some cells to be better sensors than others.
While sophisticated AI methods are needed to carry out the calculations, in the end, the insight from the studies was quite simple. To see what is happening, let us imagine an experiment where multiple people are asked to use the same scales and report their weight. While there may be some technical variability in the measurements, the observed variation across people largely reflects the variability in weights of the population. In contrast, if we ask one person to report their weight multiple times using the same scales, we will still observe some variability, which will reflect the true technical noise in the device. Previous estimates of channel capacity of signaling networks used the population variability as a measure of the technical noise in the scales, therefore grossly underestimating the accuracy of the machine.
Our MIRA award will allow us to use and develop AI methods to tackle important problems in human health. Among those, one open question remains: Why do some cancer cells die when exposed to chemotherapy while others survive?

